A Modern, Innovative Safety System
Transform case management via a unified SaaS solution powered by intelligent automation.
A Modern, Innovative Safety System
Transform case management via a unified SaaS solution powered by intelligent automation.
LifeSphere® Technology is trusted by these industry leaders:




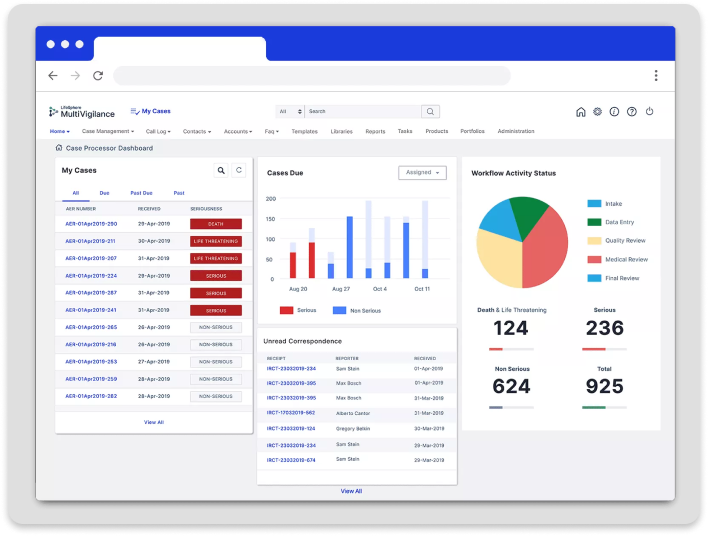
MultiVigilance
MultiVigilance powered by LifeSphere® NavaX™ is the industry’s first and leading end-to-end automated touchless case processing system, built to help safety teams worldwide achieve scalable, efficient, and harmonized case management while improving patient safety and compliance.
Significant Efficiency Gains
Accelerate case processing and reduce manual intervention via touchless case processing enabled by industry-leading automation in production today.
Comprehensive Compliance
Ensure consistent, top-quality compliance with the most robust, up-to-date support for global and regional regulations available.
Harmonized Global Operations
Establish a single global safety database with unified end-to-end workflows, secure cloud-based architecture, and open integrations.
Cost-Efficient Scalability
Make it simple to meet rising case volumes and grow as your business does with a cloud-based, multitenant platform powered by LifeSphere® NavaX™ to deliver best-in-class automation.
Features
Deliver real value with MultiVigilance.

Trusted Expertise
Utilize the industry’s preferred safety system, founded on over 30 years of safety expertise, and actively in use across hundreds of organizations globally.

Best-in-Class Automation
Leverage the only safety system with end-to-end automation in production, for significant and measurable efficiency right away, from intake to submission.

Comprehensive Compliance
Access the most robust compliance support available, with ongoing cloud updates to ensure you are always compliance with the latest regulations.

One Global Database
Establish a single global safety database founded on open architecture that enables you to seamlessly access safety data and bring together global teams and systems.

Industry Standard Practices
Deliver value right away with out-of-the-box availability of capabilities that have been developed with and are in production at leading biopharma firms.
Cloud-Based Platform
Utilize a truly multitenant platform that can readily scale to meet your needs today and tomorrow with minimal IT investment required and seamless upgrades.
Frost & Sullivan Recognizes ArisGlobal’s MedDRA Coding Agent Innovation
ArisGlobal is honored to receive Frost & Sullivan’s 2025 Global New Product Innovation Recognition for our agentic MedDRA Coding Agent. Offered standard in our AI-powered MultiVigilance offering, our MedDRA Coding Agent automates and optimizes complex MedDRA coding workflows that minimize manual burden and increase time to value for PV teams worldwide.
Frost & Sullivan Recognizes ArisGlobal’s MedDRA Coding Agent Innovation
ArisGlobal is honored to receive Frost & Sullivan’s 2025 Global New Product Innovation Recognition for our agentic MedDRA Coding Agent. Offered standard in our AI-powered MultiVigilance offering, our MedDRA Coding Agent automates and optimizes complex MedDRA coding workflows that minimize manual burden and increase time to value for PV teams worldwide.
Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved
