Regulatory Content Management
Establish a single, harmonized repository for submission content and documentation.
Regulatory Content Management
Establish a single, harmonized repository for submission content and documentation.
LifeSphere® Technology is trusted by these industry leaders:






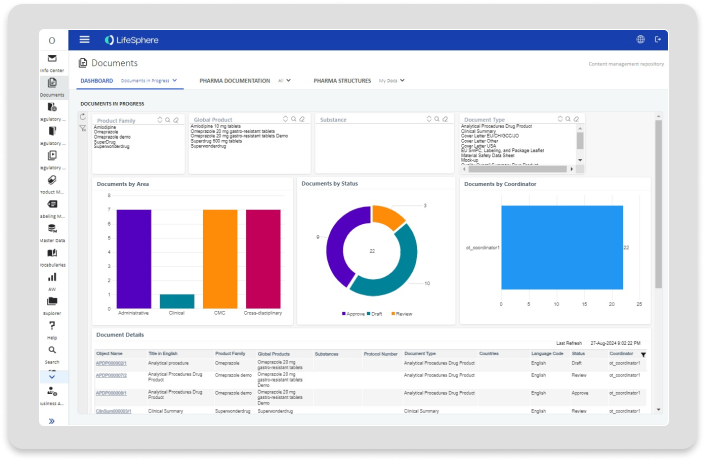
Documents
Documents gives you everything you need to manage submission relevant content and documentation up to submission readiness.
Document Control
Ensure quality and consistency with common standards, pre-configured templates, and out-of-the-box compliance support.
Efficiency Gains
Save time by making it easier to author complex documents and streamlining common document management workflows and collaboration features.
Single Source of Truth
Simplify content management establishing a single, authoritative repository for submission relevant documentation which facilitates traceable reuse of documents across submissions.
Interoperability
Accelerate submission time and cross-functional collaboration via seamless integrations with third-party repositories.
Features
Deliver real value with Documents.

Document Management
Including audit trails, version control, and template management.

Built-In Compliance
Support for requirements, including 21 CFR Part 11 compliance.

Out-of-the-Box Coverage
Get access to hundreds of pre-configured document types with relevant metadata.

Standard Workflows
Leverage controlled workflows for authors, reviewers, approvers, etc.

Submission Readiness
Built-in rendering produces high-quality, submission-ready documents, support for reusable hyperlinks and where-used feature facilitates submission lifecycle management.

Third-Party Integrations
Seamlessly integrate with key third-party systems for optimal collaboration.
Related Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved