Regulatory Planning, Tracking and Registration Management
Seamlessly coordinate regulatory information management throughout the product lifecycle.

Regulatory Planning, Tracking and Registration Management
Seamlessly coordinate regulatory information management throughout the product lifecycle.
LifeSphere® Technology is trusted by these industry leaders:






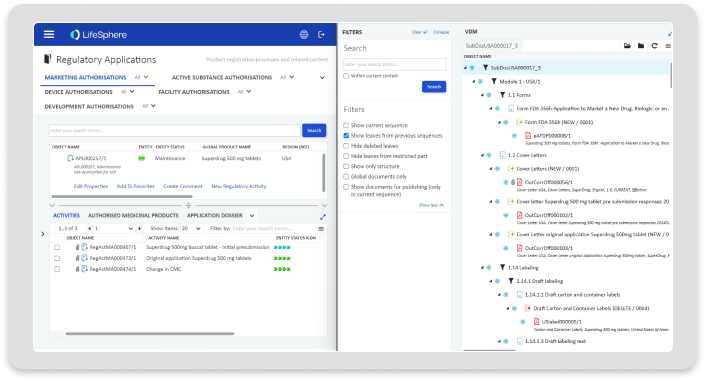
Regulatory Information Management (RIM)
RIM reduces data entry needs, improves data quality, and increases global oversight and control via robust capabilities to manage regulatory planning, tracking, and registration management.
Standardized Processes
Save time and effort by ensuring more streamlined and consistent end-to-end workflows for regulatory planning and tracking across all markets.
Enhanced Collaboration
Make it simple for your teams to work together with various stakeholders on global and national levels.
Single Source of Truth
Eliminate siloes and harmonize your operations by establishing a single area for worldwide registration activities and records.
Product Specifics
Ensure compliance with support for investigational and marketed drug/medicinal products, as well as active substances, devices, site registrations and more.
Features
Deliver real value with LifeSphere RIM.

Regulatory Planning & Tracking
Built-in support for planning your submissions, as well as tracking registrations, correspondence, commitments, and other key information.

Connectivity and Automation
Seamlessly work with your documents, submissions, interactions, labeling, product data and regulatory intelligence.

Global Coverage
Centralize global submission planning and tracking.

Localized Support
Support specific regional requirements and regulatory knowledge.

IDMP Compliance
Support for the IDMP target operating model.

Interactive Dashboards
Gain actionable insights into regulatory activities and registrations.
Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved