Submission Management and Publishing
Accelerate regulatory compliance with streamlined submissions and publishing.
Submission Management and Publishing
Accelerate regulatory compliance with streamlined submissions and publishing.
LifeSphere® Technology is trusted by these industry leaders:






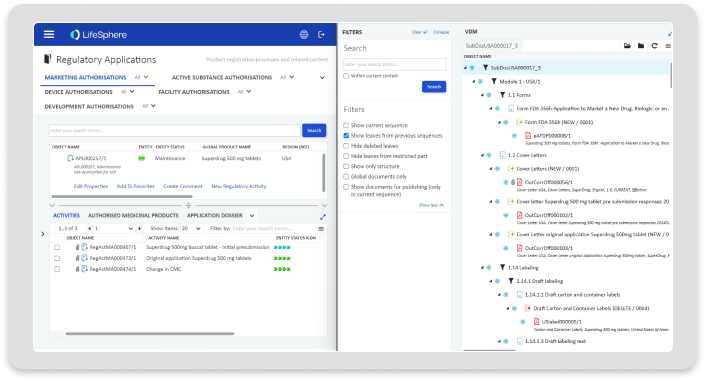
Submissions
Submissions consolidates submissions and publishing workflows in a single solution that makes it simple to create, compile, and publish submissions in any format.
Streamlined Submission Management
Leverage built-in workflows that make it simple to create, compile, and publish submissions starting from a global submission package and automated compilation of national submissions.
Worldwide Compliant Publishing
Publish national submissions in any required format, including electronic and paper publishing in compliance with HA requirements.
Unified Solution
Drive efficiencies by bringing your submissions and publishing features as part of the end-to-end RIM workflow.
Reduce Rework
Build submission templates and regulatory knowledge to reuse across different teams and regions.
Features
Deliver real value with Submissions.

Publishing Support
Support for a variety of formats, including eCTD, NeeS, PDF, and XML.

Dossier Record
Maintain the current registered dossier position and its version history.

Regulatory Knowledgebase
Safekeep knowledge on national, regional submission requirements.

Submission Reuse
Compile global submissions to use as the basis for national submissions.

Document Propagation
Readily move documents from global to local submission dossiers.

Submission Automation
Assisted compilation of submissions into predefined submission outlines.
Related Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved