Your Single Source of Truth for Quality Content
Take greater control with purpose-built, comprehensive quality document management.
Your Single Source of Truth for Quality Content
Take greater control with purpose-built, comprehensive quality document management.
LifeSphere® Technology is trusted by these industry leaders:




Quality Documents
Quality Documents enables consistent and efficient compliance with support for best-in-class capabilities through the quality document management lifecycle, including cross-domain harmonization as part of our LifeSphere Unify platform.
Standardization
Establish common standards through pre-configured documents.
Enhanced Content Management
Manage content through the quality lifecycle, including version control and audit trails.
Global Harmonization
Harmonize document management by establishing a single asset repository accessible to cross-functional and global teams and across domains via our LifeSphere Unify R&D compliance platform.
Deeper Insights
Gain strategic insights through impact assessment analysis.
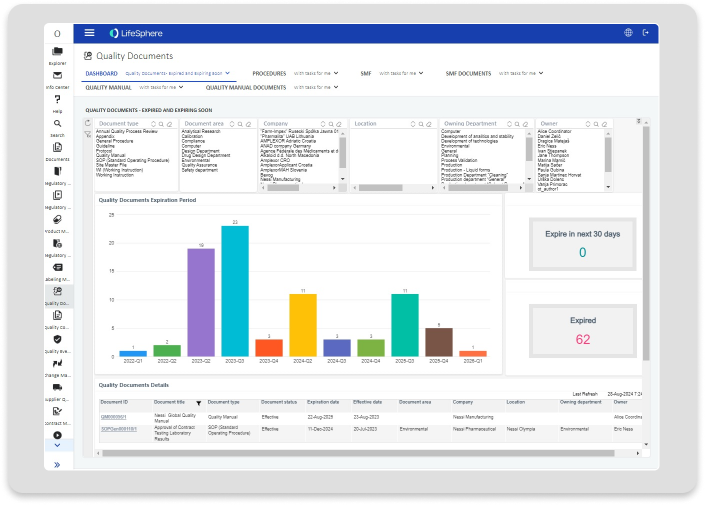
Features
Deliver real value with Quality Documents.

Controlled Templates
Provide support for high-level policies and guidelines, through specific procedures and lower-level records.

Process Standardization
Leverage predefined workflows, metadata, versioning policies, roles, and permissions.

End-to-End Document Management
Gain support across quality assurance, quality control, manufacturing, and R&D.

Data Control
Access periodic review and document validation processes, including automatic notifications.

Global Scalability
Our solution is highly suitable for global rollouts, with a proven track record of global implementations, including support for more than 18 languages.

A Single Vendor
Establish a single, end-to-end vendor by integrating LifeSphere Reporter with your larger ecosystem, including safety, medical information, and CRM systems.
Resources
Learn more about our collaborative partnership with customers.
"*" indicates required fields

ArisGlobal is a pivotal partner in the life sciences industry, specializing in solutions that drive drug development, safety monitoring, and regulatory compliance. We collaborate with more than 220 global life sciences companies, CROs, and government health authorities, including leading biopharmaceutical firms and regulatory bodies such as FDA, Health Canada, and NMPA.
Headquartered in the United States, ArisGlobal has regional offices in Europe, India, Japan and China.
Quick Links
© 2024 ArisGlobal – All Rights Reserved