Introduction
CTMS Software in Clinical Trials
A CTMS is responsible for managing all operational aspects of a clinical trial, from study startup to closeout. CTMS serves as a central information hub for study stakeholders to effectively plan, implement, manage, and analyze their entire clinical study portfolio.
Clinical trials are complex. Biotech, pharmaceuticals, and other research-focused life sciences organizations run highly complicated clinical trials in the search for new medicines, devices, and the ultimate cure for many of the world’s diseases. Using software helps you manage clinical trials in clinical research.
LifeSphere CTMS Software is an easy-to-use clinical trial management system that helps clinical operations teams accelerate timelines, stay organized, and reduce complexity. If you are interested in learning more, you can schedule a demo.
Accelerate Clinical Trials with LifeSphere CTMS. Reduce study timelines with a modern, easy-to-use cloud application that streamlines study management and also automate key activities across the trial lifecycle
Our All-in-One CTMS Software will help streamline your clinical environment with a turn-key platform for operations that includes payments and monitoring and delivers a seamless connection with LifeSphere eTMF
Unifying ClinOps and Data Management in the Cloud
Unified with the LifeSphere Clinical cloud platform – including LifeSphere eTMF, LifeSphere EDC and LifeSphere EasyDocs – to seamlessly connect operations with data management.
What Are Some Common Benefits of LifeSphere CTMS?
With LifeSphere CTMS, you will be able to easily improve efficiency, productivity, and cost savings.
Below are some of our benefits of our CTMS:
- Create a single source of truth for all clinical trial operations on an interoperable, cloud-based platform.
- Replace manual workflows and lower your cost of ownership using intelligent automation workflows.
- Proactively manage and mitigate risks before they impact trial timelines and budgets.
- Facilitate smooth site interactions with out-of-the-box site payment and monitoring capabilities (shown to decrease site monitor times by 30%).
- Empower teams to autonomously conduct trial operations using an intuitive platform powered by smart automation.
- Unlock real-time insights with end-to-end access to clinical trial data and reporting.
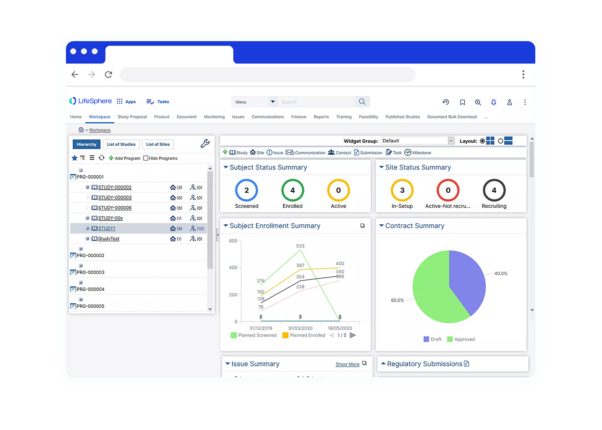
Centralized and Globally Accessible CTMS Software
CTMS Software allows sponsors to be more effective, to make improved decisions, to establish compliance, to select investigators well, to monitor patient recruitment and to manage finances. LifeSphere CTMS includes comprehensive directories to manage site personnel (investigators, sub-investigators, study coordinators, pharmacists) and organizations (hospitals, competent authorities, CROs, suppliers).
LifeSphere CTMS provides functions for tracking patients, patient visits, EDC data, activities, queries, deviations, adverse events, drug supply, monitoring visits and finances. A comprehensive dashboard provides total visibility into the status of each study, country, site and investigator to provide the information necessary to make the best decisions possible.
Simplified Trip Reporting
Supports report app authoring, review, and approval for large field teams.
Other benefits include increased case processing quality and error reduction thanks to natural language processing and cognitive computing.
Learn more about our production-ready automation capabilities.
Site Monitoring Planning
Enables monitors to efficiently conduct and document monitoring visits with an automated review and approval process governed by user-defined workflows and event-based planning.
Flexible Payment Modules
Included payment module allows for hassle-free configuration of payment terms so teams can easily pay as contracted.
Resources
An Easy and Intuitive CTMS for Organizations of All Sizes
LifeSphere CTMS10 is a core offering in LifeSphere Clinical’s unified cloud platform.
LifeSphere CTMS10 is an end-to-end solution that makes the entire clinical trial management process easier and more transparent for companies of all sizes.
The Evolving Role of CTMS in Risk Management
Clinical trials are a necessary and critical activity in releasing new, and often-times lifesaving, drugs and medical devices to the market. To achieve success, however, in the face of rising development costs and fewer approvals, life sciences organizations are under severe pressure to manage these trials in a way that is risk-averse and cost-effective.
The Benefits of a Unified Clinical Platform
For nearly 20 years the issue of disconnected, poorly integrated systems and processes has hampered clinical R&D. The problem and its challenges have been discussed, dissected, and top of mind of most clinical operations leaders. The constant and critical push to accelerate a product’s time to market continues to push clinical teams to seek solutions that deliver greater efficiencies. After 20 years of point-to-point integrations, the time has come for a different, advanced, and unified approach.
The Clinical Domain is Evolving. Make Sure Your CTMS is Evolving With It
The Evolving Role of CTMS in Risk Management takes a broad look at the challenges facing clinical trial management, how the clinical domain is changing, and how the industry can better respond.

